Captisol has enabled products based on different molecular classes (neutral, charged, MWs between 236-808), across therapeutic areas (antiviral, oncology, antifungal, CNS, more), and in varied presentations (ready to use parenteral solution, concentrate, lyophilized powder in a vial, oral liquid). This 20+ year clinical, regulatory & safety record streamlines the approval process for Captisol-enabled product candidates.
What is Captisol?
By significantly improving the solubility, stability, and therefore bioavailability of insoluble active pharmaceutical ingredients (APIs), Captisol is an ideal excipient for difficult to formulate compounds. Partners have successfully used Captisol to overcome formulation issues to enable commercially successful products for the past twenty years, with additional approvals on the near horizon.
Captisol Chemistry
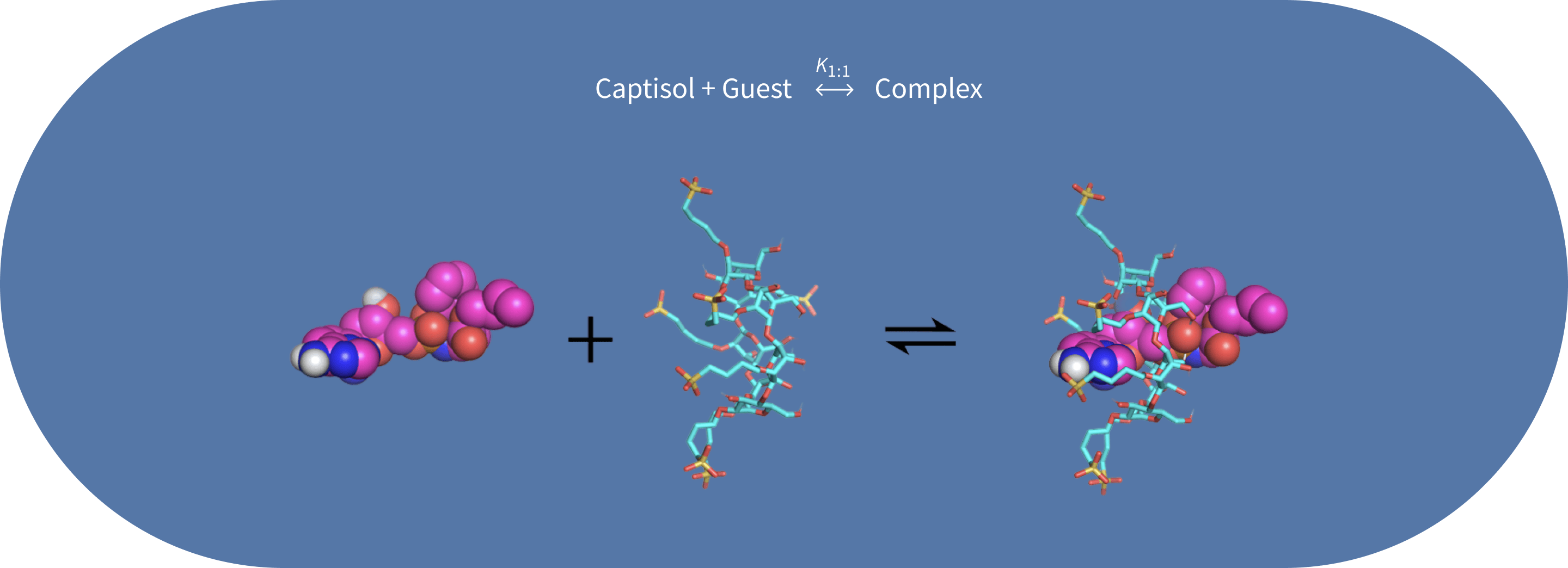
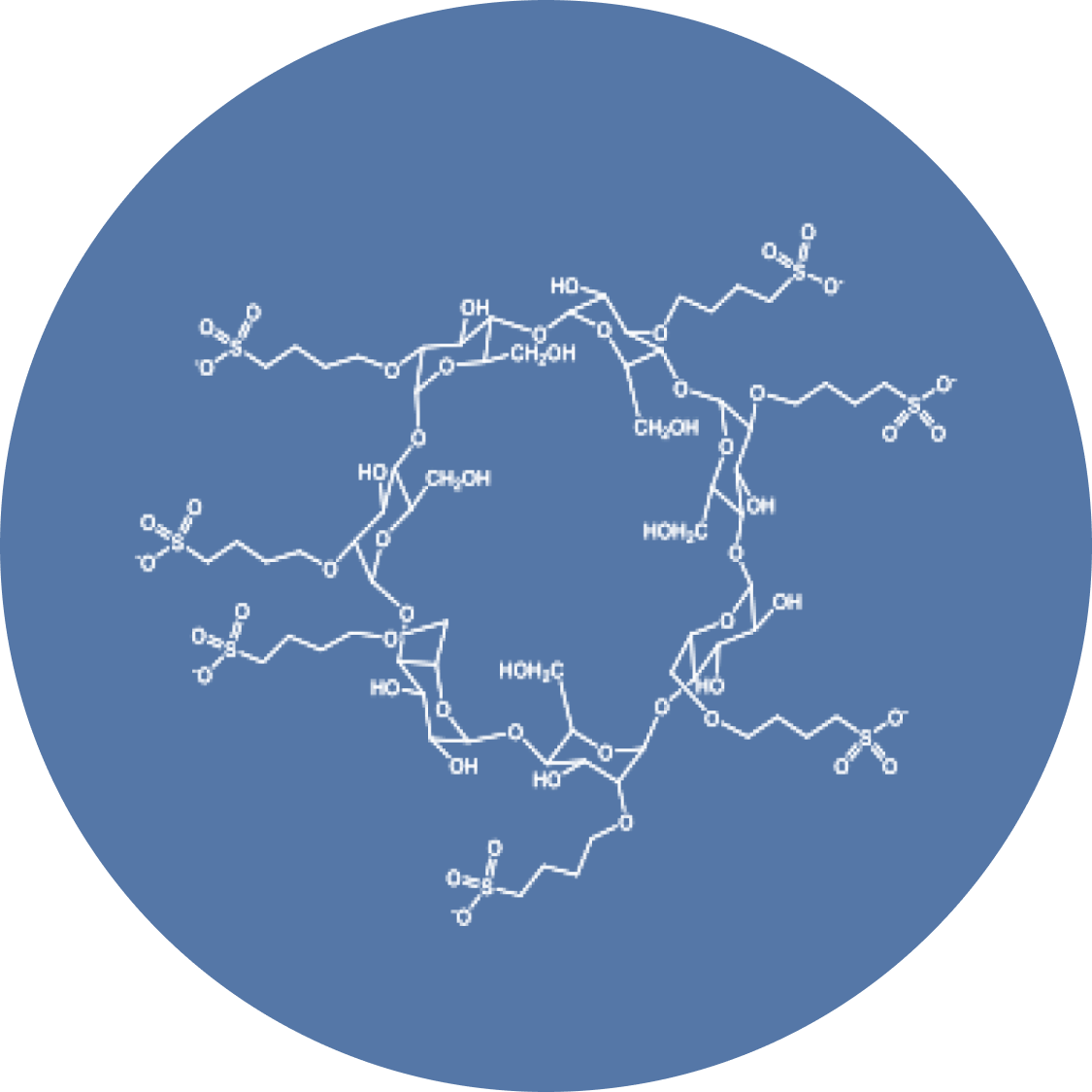 Cyclodextrins (CDs) are cyclic oligosaccharides typically containing 6(α-CD), 7(β-CD), or 8(γ-CD) glucopyranose units, oriented in a truncated cone structure with a hydrophilic on the exterior and a lipophilic interior. This lipophilic interior can then partially or fully contain an insoluble guest molecule, forming a cyclodextrin complex with better solubility and/or stability than the guest molecule. Typical α-, β-, and γ-cyclodextrins (particularly β) have limited aqueous solubility and show toxicity when given by injection, so Captisol® was derivatized from a β-cyclodextrin to have ~6.5 sulfobutylether groups per cyclodextrin molecule, to generate a parenterally safe CD-derivative.
Cyclodextrins (CDs) are cyclic oligosaccharides typically containing 6(α-CD), 7(β-CD), or 8(γ-CD) glucopyranose units, oriented in a truncated cone structure with a hydrophilic on the exterior and a lipophilic interior. This lipophilic interior can then partially or fully contain an insoluble guest molecule, forming a cyclodextrin complex with better solubility and/or stability than the guest molecule. Typical α-, β-, and γ-cyclodextrins (particularly β) have limited aqueous solubility and show toxicity when given by injection, so Captisol® was derivatized from a β-cyclodextrin to have ~6.5 sulfobutylether groups per cyclodextrin molecule, to generate a parenterally safe CD-derivative.
These sulfobutylether groups make Captisol® unique:
- The very low pKa of the sulfonic acid groups results in multiple negative charges at physiologically compatible pH values, greatly increasing the solubility & safety of the cyclodextrin & provides another "handle" by which molecules can interact with Captisol.
- Captisol can increase water solubility by 10x to 150,000x depending on compound structure, concentration of Captisol®, pH effects on the extent of drug ionization, buffer selection and strength of complex.
- Aqueous formulations with Captisol are often simpler & less toxic than the use of organic solvents, surfactants, and extreme pH conditions to achieve desired dosages.
- Stability of drugs in aqueous solution may be improved on with Captisol. Enclosure of the labile area of the drug in the cyclodextrin cavity or interaction with the SBE substituent can reduce decomposition rate by ‘hiding’ the reactive center.
- The four-carbon butyl chain coupled with the repulsion of the end group negative charges allows for an "extension" of the cyclodextrin cavity, often resulting in stronger binding than other modified cyclodextrins.
- Complexation is a non-covalent, reversible interaction typically occurring in a 1:1 stoichiometry.
Captisol-Enabled Products Approved 2002-2024
VFEND
Voriconazole
Pfizer
Antifungal
IV Administration
Lyophilized Powder
GEODON IM
Ziprasidone mesylate
Pfizer
Antipsychotic
IM Administration
Lyophilized Powder
ABILIFY
Aripiprazole
BMS
Antipsychotic
IM Administration
Ready to Use Solution
CERENIA
Maropitant citrate
Pfizer
Antiemetic
Veterinary SQ and IV
Ready to Use Solution
NEXTERONE
Amiodarone
Baxter
Antiarrhythmic
IV Administration
Premixed IV Bag
Captisol replaced polysorbate 80 and benzyl alcohol in original formulation
First product with Captisol in a premixed IV bag
KYPROLIS
Carlfilzomib
Amgen
Multiple Myeloma
IV Administration
Lyophilized Powder
NOXAFIL
Posaconazole
Merck
Antifungal
IV Administration
Solution Concentrate
EVOMELA
Melphalan
Acrotech
Multiple Myeloma
IV Administration
Lyophilized Powder
CARNEXIV
Carbamazepine
Lundbeck
Antiseizure
IV Administration
Solution Concentrate
BAXDELA
Delafloxacin meglumine
Melinta
Antibacterial
IV Administration
Lyophilized Powder
In development, Captisol was selected over Solutol not only to improve solubility, but also to improve stability (freezing not required) and to lessen venous irritation.
ZULRESSO
Brexanolone, Allopregnanolone
Sage
Postpartum Depression
IV Administration
Solution Concentrate
VEKLURY
Remdesivir
Gilead
Antiviral
IV Administration
Solution Concentrate and Lyophilized Powder
Treated 13 million people in 170 countries
For use in pediatric patients from birth
Approved for patients will all levels of renal impairment
SESQUIENT
Fosphenytoin
Sedor
Antiseizure
IV Administration
Solution Concentrate
Captisol solubilizes phenytoin (active) that is a degradant of the fosphenytoin prodrug
Captisol increased the room temperature stability of the formulation
DOCIVYX
Docetaxel
Ingenus Pharmaceuticals
Antineoplastic
IV Administration
Solution Concentrate
Product with Captisol does not need intermediate dilution step prior to addition to infusion solution
No polysorbate 80 in product
MEKINIST(Oral Solution)
Trametinib
Novartis
Melanoma/Glioma
Oral Administration
Powder for Oral Solution
First product with Captisol for oral administration
FYCOMPA IV
Perampanel hydrate
Eisai
Antiseizure
IV Administration
Lyophilized Powder
Initial product approval in Japan
Lasix® ONYU
Furosemide Injection
SQ Innovation
Edema
Subcutaneous Administration
Solution Concentrate
Safety
Captisol is eliminated by glomerular filtration in the kidneys. Two decades of patient experience and recent data have shown that Captisol is safe, even in patients with severe renal impairment. Clinical studies were performed in renally compromised patients on BAXDELA® (a Captisol-enabled product). Conclusions from these studies stated that "Increased SBECD exposures did not result in any noticeable increase in drug-related TEAEs in this study." And "... decreasing renal function causes reduced SBECD (Captisol) clearance and increased exposures, but SBECD continues to exhibit a good safety and tolerability profile in IV formulations."
In another Captisol-enabled drug product, Gilead performed clinical safety studies for VEKLURY® in patients with moderately and severely reduced kidney function. The results of the study were favorable and in August 2023 the package insert for Veklury® was changed to state that "No dosage adjustment of VEKLURY® is recommended for patients with any degree of renal impairment, including those on dialysis..."
Read Gilead ArticleRecent Client Projects
- Improved solubility and stability for administration by slow subcutaneous infusion
- Formulated poorly soluble/unstable compound and worked with third party to prepare spray-dried materials for pre-clinical studies

